It turns out the whole world hasn’t been hanging on my every word! Who knew? What follows is a short version of how we got to where we are at the moment…
On May 15, 2013 Seaside Therapeutics, Inc., the sponsor of a drug trial my 9 year old son Caleb is participating in, sent a communication to clinics involved in the administration of the Phase III study stating the following:
“We regret to inform you that Study 209FX303 [An Open-Label Extension Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of STX209 (arbaclofen) in subjects with Fragile X Syndrome] is being terminated immediately. The closure of the study is due to resource limitations at Seaside Theraputics, Inc., and is not related to any known safety issues in patients dosed with STX209.”
Thus began our latest challenge. In case you’re a first time visitor, Caleb has fragile X syndrome. Fragile X syndrome (FXS) is the most common known cause of inherited intellectual disability. It is caused by the mutation of a single gene. A single gene that controls the production of a protein referred to as FMRP. This protein, most commonly found in the brain, is essential for normal cognitive function.
As the name implies, the mutation is carried on the X chromosome. I passed along my X chromosome with the unstable expansion. The risk of my passing on that chromosome (since I have two of them and only one is unstable) was 50/50. The risk of that unstable chromosome expanding to a “full mutation,” wherein the gene is turned off and the FMRP is not made, was nearly 100%. I had no idea I was a carrier, I was unwittingly playing Russian roulette with my child’s life.
I lost.
We first noticed that my son had delays when he was 12 months old and not babbling. He was eerily silent except for crying or his amazing belly laughs. We were referred to our local Early Intervention program, which provides services to children, under age 3, with delays. Caleb was 16 months old when he began receiving services. We were all expecting that he would catch up to other kids his age with a little extra help.
Then at age 22 months, we received the news that he had fragile X syndrome. We now knew that he wouldn’t catch up, in fact, he would continue to lag further and further behind his peers. There were no drugs approved for treating fragile X, and there still are not, but even then researchers were optimistic that they could find some sort of targeted treatment for the underlying mechanism. That was 7 long years ago.
Seaside Therapeutics, based in Cambridge, MA, began testing a drug that would do what researchers had long hoped to accomplish. The results from the double blind placebo controlled study were so promising that parents begged for and were granted an extension trial. During the extension each child would take the actual drug and further gains or side effects would be monitored.
When the first extension trial reached its end, desperate parents requested that the company continue to make this drug available to their children. Seaside told parents they would continue the extension trials until FDA approval was granted. Parents were able to take a breath, the drug was working and the supply would continue.
My son participated in the Phase III double blind, placebo controlled study last fall. In January we signed up for a 25 month extension. We were told that if the Phase III results, due the fall of 2013, were positive, they would seek FDA approval to market STX209. I noticed changes in my son both during the trial and the extension. I noticed increased eye contact and more speech most clearly. I was afraid to believe in the changes, I was concerned that I had fallen victim to the placebo effect and was seeing only what I wanted. During one of our many, many school meetings, his teacher and several of his therapists made observations that backed up what I was seeing. I was elated.
My son continued to improve over the spring, we did things I never thought we would do. We flew together to IL for a fundraiser, leaving his father behind, and he did fantastic. He enjoyed himself so much that he has been asking to go back since we got home. During the trip he was meeting a lot of new people; he spent a great deal of time in crowded situations. This is where I noticed that his social anxiety, the primary target for this trial, was so much improved.
He began playing with toys appropriately and not just imitating what he had seen on TV or with other kids. He began planning, he searched a Toys R Us catalog, found a NERF gun he wanted and asked for it. Let me rephrase, he begged for it. When I finally caved and let him have the NERF gun, he was able to navigate the store and search through 4 separate NERF displays to locate the gun he wanted. He did this independently. He began answering direct questions appropriately. Whereas he used to throw out any answer or word when asked a question just to stop the questioning, he now answers the question. Not always the way I’m anticipating but this new skill has allowed him to show off his sense of humor too.
Some ask me, how do I know that the changes aren’t the result of normal development? I don’t know, I suppose, not for certain, but his improvements have come on so quickly it would seem terribly coincidental that they just happened to occur after he started this drug. I wrote about his amazing progress here. Then in April, an article I wrote was published in the Bay State Parent Magazine, a local monthly. The article, “Hoping For Too Much?” shared the highlights of our experiences to that point. I ended that article with this:
“He’s changing faster than I can dream new dreams.”
And now, after 4 months of ever growing dreams, it is all being taken away. I’ve been working with some of the parents of the over 350 kids on this cancelled extension trying to find out why this happened, how this happened but, more importantly, how do we stop it from happening?
We have lobbied politicians, we have reached out to local and national media outlets, we have reached out to the company and our friends. We have gotten nowhere. We are still facing having to give up a medication that has been shown to be effective in children with fragile X (that is what the Phase II trial is for) when there are no other alternatives on the market.
There are other drugs being studied but not every child qualifies for these other studies. Besides that, how many times should a young child have to go through one of these trials? How many times do we have to pin him down for blood draws, put him through the stress of evaluations and miss school? It was so hard to sign up for this trial, but I did it out of hope that good would come of it. The sacrifices were worth it to keep research moving forward and to give our son a chance to shine as we had watched with other participants.
Now I feel that those 4 months were a tantalizing taste of what could be, it feels so cruel to take it away from him and from our family. Just imagine how hard it will be to watch him struggle with things he can do now. How hard will it be to watch him struggle and know there is something out there, beyond our reach, that would help him?
I don’t yet know what is next. I’m holding on to a slim hope that if we continue reaching out and publicizing the situation, we may be able to convince Roche Pharmaceuticals, which previously provided funds to Seaside, to provide the money needed to reinstate the extension trial, to complete the rest of the necessary phases of the trial and get the drug on the market. I cannot yet give up on that hope.
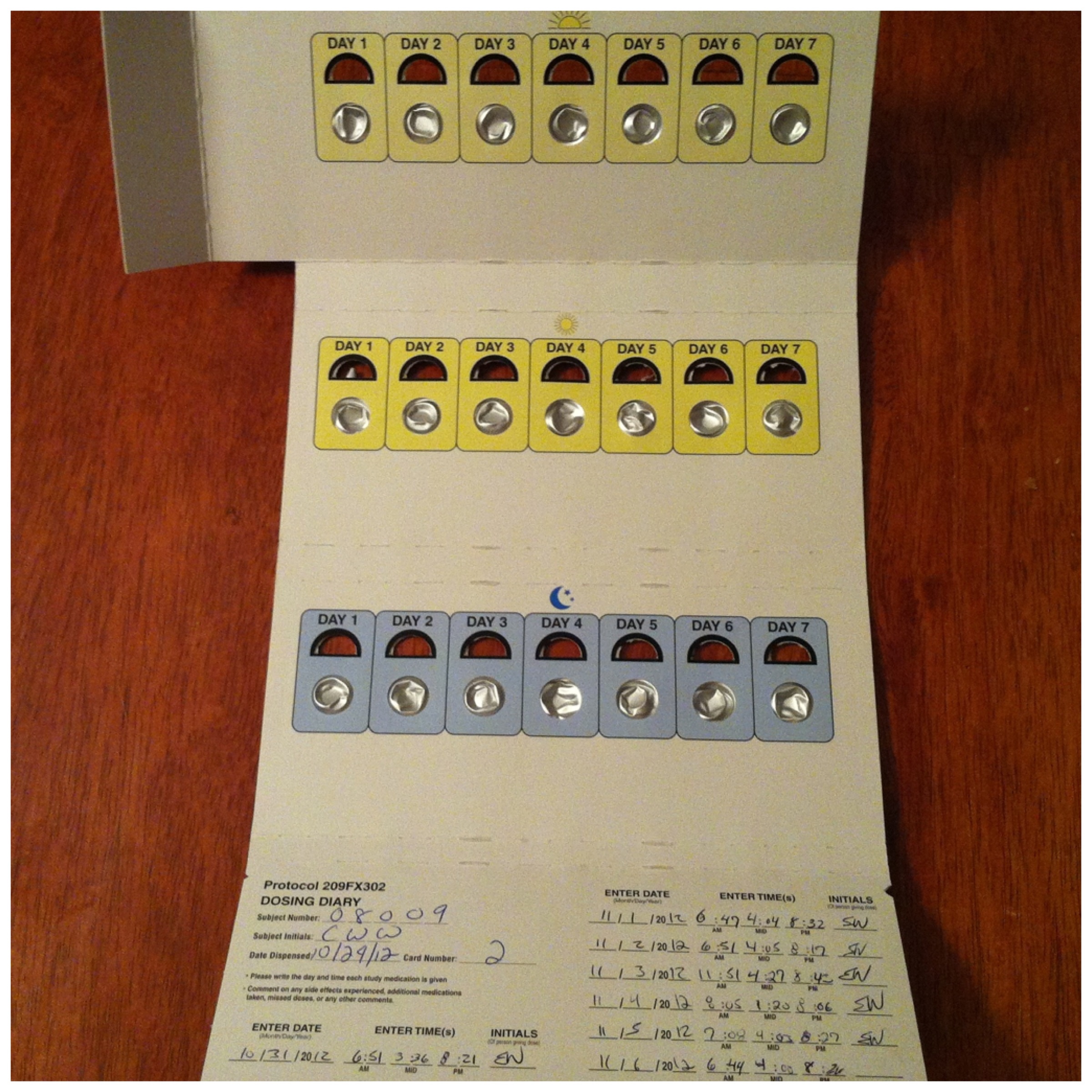
 Every day as I hand him the pills I dread the day when I’ll have to stop. That day is fast approaching. I don’t know how to explain to him that Seaside and Roche didn’t think he was worth the cost. How do I explain any of this to him? He’s NINE, he’s a baby! I can’t explain any of this to him because it doesn’t make any sense. This just shouldn’t be allowed, not here in a country with so many resources.
Every day as I hand him the pills I dread the day when I’ll have to stop. That day is fast approaching. I don’t know how to explain to him that Seaside and Roche didn’t think he was worth the cost. How do I explain any of this to him? He’s NINE, he’s a baby! I can’t explain any of this to him because it doesn’t make any sense. This just shouldn’t be allowed, not here in a country with so many resources.